Atoms bond together with valence electrons.They could share them equally, share them unequally or completely steal them. When they share them equally it is called a
Covalent bond. When they share them unequally it is called a
Polar Covalent bond. When they completely steal them it is called a
Ionic bond. Some examples for Covalent, Polar Covalent and Ionic: Oxygen-Oxygen bond in O
2 , Oxygen-Hydrogen bond in H
2O and the Sodium-chlorine bond in NaCl respectively. In Polar bonds there is a Slightly or partially positive side and a slightly/partially negative side. The name Ionic comes from the word
ion, Which is a charged species ( in Chemistry this could be a atom , molecule or any other particle. But when you get into Biochemistry you have a problem
: ), it supposedly has nothing to do with the Ionic column ;). There are 2 different types of Ions: Cations and Anions. The Cations are positively charged ( and are formed by taking away electrons ) and the Anions are Negatively charged ( formed by adding electrons ) . Ionic compounds ( I will tell you what that is next) are formed by Ionic bonds ( which are formed by electrostatic forces pulling the cations and anions together) .
A molecule is 2 or more atoms bonded, while a
compound is a molecule with different elements in it. The O
2 above is a molecule but
not a compound. The H
2O ( which you might recognize as water ) and the NaCl are molecules
and compounds. Intermolecular forces are forces between
molecules , while intramolecular forces are forces between atoms in a molecule ( these are also called bonds ). There are 3 main types of intermolecular forces and some others, London Dispersion forces, Dipole-Dipole interactions and Hydrogen bonding ( which is really an extra strong dipole-dipole interaction , and not a bond at all since its inter- ). Dipole-Dipole interactions/forces are attractions between polar molecules, the Partially positive side of one molecule is attracted to the partially negative side on another molecule, as in H-Cl. Hydrogen bonding is ( like I said ) an extra strong type of dipole-dipole forces, which only happens with a hydrogen on an oxygen , Fluorine or nitrogen and another partially negative atom on another molecule. Hydrogen bonding takes place in water and is responsible for the surface tension on the water's surface. Hydrogen bonding is extremely strong ( when I say extremely I mean its stronger than regular dipole-dipole , but not stronger than ion-ion forces ) because Hydrogen has only one electron and it can go on one side while a small partially negative atom ( of another molecule ) can creep up close to the positive nucleus of Hydrogen on the other side. London dispersion forces are the weakest of the three IMFs that I named. But they are dependent on the polarizability of the molecule , which is dependent on the size of the electron cloud . If you have a very large electron cloud molecules , and hence some very polarizable ones you would have strong LDFs. Anyway London Dispersion forces ( LDFs) are instantaneous forces that happen when tat one instant the electrons in a molecule are farther to one side and then it has momentary poles , this induces other molecules around this molecule to have momentary poles and then the molecules are attracted. But this isn't permanent and happens only for a moment, then the electrons go regular again . This happens over and over again, so a force of attraction is seen , but the polarity is not permanent and the force is generally weak ( when you got molecules with large electron clouds it is not so weak ). Every group of molecules have this force, but only in ones where the molecules atoms have very similar electronegativities , LDFs are the only IMF. Intermolecular forces are related to boiling points, The stronger the force the higher the boiling point. This is because IMFs keep molecules together and to boil liquid you need to make the molecules "fly" apart from each other.When you compare Cl
2 & HCl, Cl
2 has a higher boiling point, even though HCl has Dipole-Dipole while Cl
2 has only LDFs. Like I said LDFs are stronger with a larger electron cloud ( which makes it more polarizable , since there is a higher chance of that moment when the electrons are unequally shared ) , and in this case the larger and more polarizable cloud of Cl
2 makes its LDFs stronger than the LDFs and Dipole-dipole of the smaller HCl. The reason that oil and water do not mix is that the oil only has LDFs and the water has H-bonding. Even though the oil molecules are more attracted to the water ones, the water is more attracted to itself and does not let the oil in. The way to determine if a molecule is polar and hence if it has a dipole is too use Electronegativity difference in bonds ( to determine if they are polar, since if the bonds aren't polar the is no way the molecule can be ) and to then use ( if there are polar bonds ) Valence Shell Electron Pair Repulsion theory ( or VSEPR ) to determine if the polar bonds ( the dipoles ) cancel. If they do cancel then the molecule is not polar and it does not have a dipole. If they do
not cancel , and add up to a net force one way, then the molecule is polar and has a dipole. Now there is more than one way to find molecular shapes, but AP chemistry uses VSEPR theory and this is what I will write about for molecular shape. Now , the theory is that electron pairs repel each other since they are both negative ( We are talking about electrons in the valence shell since they are the ones in bonding ). Bonds are pairs of electrons and they repel each other. There are also free pairs that do not bond, these also repel each other and bonds. So to be able to use VSEPR theory you need to be able to draw Lewis dot diagrams. These are basically diagrams that only show the valence electrons and show how they are used in bonding. Lets do HCl,
First you draw the placement , in this case its easy
H Cl
Then you draw the bond(s)
( in this case there is only one bond , H can only make 1 bond since it has only 1 valence electron )
H
-Cl
Then you fill in any missing electrons as dots, Cl has 7 valence and the line of a bond is 2 so there are 6 missing around the chlorine
..
H
-Cl
:
''
well its a bit hard to do it on the computer, but on paper its easy.
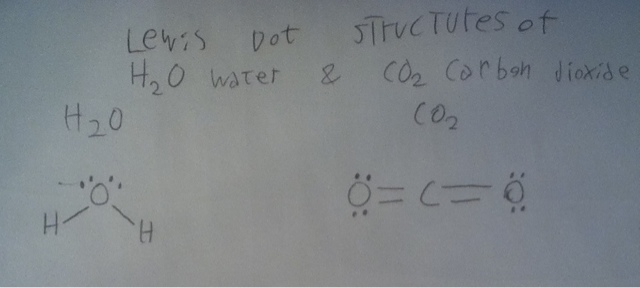
Now VSEPR has many shapes , but most of them are based on 5 common shapes ( these are the only ones in AP Chemistry , but they are more like 8 real ones and infinite theoretical ones ) Linear, Trigonal Planar, Tetrahedral ,Trigonal Bipyramidal and Octahedral
no free electron pairs
| 1 free e- p
| 2 f e- p
| 3 f e- p
| 4 f e- p
| 5 f e- p
|
Linear ( there is not anything based on this, its like HCl , one atom bonded to another )
Trigonal Planar Bent Linear - - -
Tetrahedral Trigonal Bent Linear - -
pyramidal
Trigonal Bipyramidal Seesaw T-Shaped Linear Linear -
Octahedral Square Square T-Shaped Linear Linear
pyramidal planar
 |
| List of some VSEPR theory shapes |
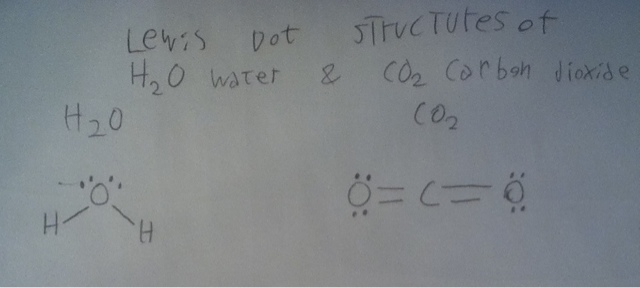 Now VSEPR has many shapes , but most of them are based on 5 common shapes ( these are the only ones in AP Chemistry , but they are more like 8 real ones and infinite theoretical ones ) Linear, Trigonal Planar, Tetrahedral ,Trigonal Bipyramidal and Octahedral
Now VSEPR has many shapes , but most of them are based on 5 common shapes ( these are the only ones in AP Chemistry , but they are more like 8 real ones and infinite theoretical ones ) Linear, Trigonal Planar, Tetrahedral ,Trigonal Bipyramidal and Octahedral
No comments:
Post a Comment